Wednesday, December 15, 2010
Jeopardy!
There was an exciting tie for first place. Malcolm, Gabriella, Paul & Edo's team tied with Jose, Adam and Jonatan's team. First place receives a no homework pass and a late homework pass.
Coming in third place were: Francesca, Ivan, Tomi and Antonella. Third place receives a late homework pass.
Monday, December 13, 2010
Film Reflection
1. What is the most memorable thing you learned from the film so far?
2. If Al Gore were sitting with you right now, what question would you ask him?
3. Based on what you have watched, what is your vision for the world ten years from now?
4. Based on what you have watched, what is your vision for the world one hundred years from now?
5. What are three things you learned from the film that you didn't know before?
6. Pick one thing from the film and describe how it relates to something we have learned about so far this year.
Sunday, December 5, 2010
Acid Rain Rubric
 Click the picture of the rubric to make it print size. You will receive this rubric in class tomorrow to give yourself a 'pregrade' on your project.
Click the picture of the rubric to make it print size. You will receive this rubric in class tomorrow to give yourself a 'pregrade' on your project.
Final Taping of Acid Rain Videos
On Tuesday we will be wrapping up the acid rain experiment.
On Wednesday we will watch the videos in class. Make sure you either have your video on a flash drive or bring a computer to class so we can hook it up to the projector to watch it.
Your peers will grade your video as well. Lastly, you will complete a peer assessment where you give your group members grades for their participation and teamwork.
Thursday, December 2, 2010
Acid Rain Links
Francesca found this great one which is an overview of acid rain and what you can do to help minimize its effects.
Wednesday, December 1, 2010
Acid Rain PSA
Your video should include:
1. A clear explanation of acid rain. What is it? How is it formed? What are the two most common compounds that contribute to acid rain?
2. How does acid rain affect plants, animals, aquatic ecosystems, the soil, man-made objects and people?
3. You can pick a specific country and focus on the effects of acid rain in Switzerland, Russia, Germany, the U.S. etc.
4. What can people to do minimize acid rain?
The plan for working on the video:
Wednesday Dec. 1
Go to the computer lab to research additional information and work on your script.
Friday Dec. 3
Turn in script for proof reading, rehearse, create props/posters for the video
Homework over the weekend:
Practice! Practice! Practice!
Monday Dec 6
We will video tape the PSA's in class. These video's will be posted on the blog.
Creativity Counts! This will serve as your test on acid rain, so make sure your information is correct. Everyone must participate in the project.
1. Content: Is the content correct? Did you answer the questions above? (60%)
2. Team Work: How well did you work with your teammates? Did each person contribute? You will be grading each other on participation. (20%)
3. Creativity: Is your project a snooze-fest or does it inspire people to make a change? (20%)
Tuesday, November 30, 2010
The Effects of Acid Rain
Homework: Write a three paragraph summary IN YOUR OWN WORDS on the effects of acid rain on the soil, lakes/rivers, fish, people and man-made objects. You should use the website from last night's homework as well as the notes from class today. Click on any of the images below to get to a larger version of the text. This is due tomorrow in class. You may type or hand write this assignment. The information below is from the Delaware Department of Resources & Environmental Control





Monday, November 29, 2010
Acid Rain Data Day 1
Homework: Read about acid rain here and take notes for a reading quiz.
Wednesday, November 24, 2010
Climate Change Experiment
Homework: Find an article on climate change or global warming and write a summary of the article. Your summary should be at least five sentences long. The article should be from 2009 to the present.
This is due when we return from Thanksgiving break.
Monday, November 22, 2010
Lab Report Due Today & Climate Change 1
We started to talk about climate change and what that means. You will be the ones to inherit this planet, the state it is in is up to YOU!
Since you worked so hard on your lab reports, you do not have homework tonight.
Thursday, November 18, 2010
Test Tomorrow & Homework Over the Weekend
After you finish your test, you need to read the packet on Climate Change and answer the questions. These questions are due on Monday.
Wednesday, November 17, 2010
Writing a Lab Report - Using Excel
Click through the PowerPoint below to see how to use excel to make a graph.
One last reminder: you have a test on Friday and your lab report is due on Monday.
Tuesday, November 16, 2010
Writing a Lab Report
Homework: Start a rough draft of your lab report. Due tomorrow are the Title, Introduction and Methods & Materials sections.
A few things to remember:
Please do not use "I" or the word "prove" in your lab report.
Yes, spelling counts for this assignment.
Write in the past tense (was, were, --ed)
Monday, November 15, 2010
Finishing up Molecules & Temperature
Make sure you proof read your work and write neatly! This assignment counts for two lab grades.
Friday, November 12, 2010
Seeing Molecules & Temperature Investigation 2
 Today we started a more formal experiment to collect data and continue to see the effect that temperature has on the movement of molecules.
Today we started a more formal experiment to collect data and continue to see the effect that temperature has on the movement of molecules.On Monday we will finish the experiment.
On Friday, November 19th you will have a test on The Air Unit. Things that will be on your test include: the composition of the atmosphere, layers of the atmosphere, what causes wind, how temperature affects the movement of molecules, how temperature is related to hurricanes, as well as some science skills like graphing and interpreting data.
Thursday, November 11, 2010
Seeing Molecules & Temperature Investigation
 On Wednesday we did a practice investigation to see how temperature affects the movement of molecules. Students had some good suggestions on ways to reduce the sources of error in the experiment. Taking what we learned, we'll do the experiment on Friday.
On Wednesday we did a practice investigation to see how temperature affects the movement of molecules. Students had some good suggestions on ways to reduce the sources of error in the experiment. Taking what we learned, we'll do the experiment on Friday.Homework: none.
Tuesday, November 9, 2010
Seeing Currents
Homework: You will have an open notes quiz tomorrow.
First read the paragraphs below about convection. Then read about atmospheric circulation here.
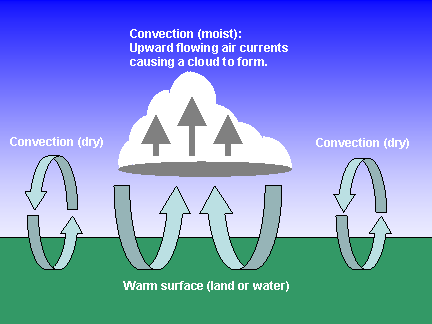
The one way that heat is transferred from one place to another is convection. Convection takes place when heated molecules move from one place to another, taking the heat with them. Convection is common in both the atmosphere, as well as in the oceans.
Heated air in our atmosphere expands, becoming less dense. Because it is less dense, it rises upward. Cooler air rushes in to replace the air that lifted up. As warm air rises, and cool are falls, a giant circular pattern is created. Eventually the warmer air cools, and begins to fall again. [1]
| "Convection" has several, related meanings in weather....but it always involves rising air. It usually refers to "moist convection", where the excess water vapor in rising air parcels condenses to form a cloud. The heat released through this condensation can help to sustain the convection by warming the air further and making it rise still higher, which causes more water vapor to condense, so the process feeds on itself. Convection can also be dry, as occurs on a sunny day over the desert. The sun warms the ground, and convective air currents help to remove the excess heat from the surface. Dry convection also occurs during the day even when clouds are forming...you just can't see it. |
| Interesting facts: |
| MAKING THE EARTH LIVEABLE: Convection (both dry and moist) help to make the Earth liveable by removing excess heat from the surface, and transporting it high into the atmosphere. It has been calculated that, without convection, the average surface air temperature on the earth would be about 125 deg. F, rather than the current 59 deg. F. [2] |
Monday, November 8, 2010
Windy Weather
To learn more about how these storms are formed we watched and took notes on the video clip below.
Homework: Click the picture below to link to the animation we started to go through in class. Take notes, you will have an open notes quiz on this information tomorrow.

Thursday, November 4, 2010
Weather & Wind
Below is your homework:
Find a news article from 2008 to the present one of the following topics:
Hurricanes
Tornadoes
Cyclones
There are many news sources out there, these are just a few suggestions :)
www.sciencedaily.com
www.bbc.co.uk
www.boston.com
For the article complete the following on a sheet of paper. If you type your answers, you MUST bring a printed copy to class.
1. What is the title of the article?
2. When was this article published?
3. What news source is this article from AND who is the author?
4. Write a 5 sentence summary of the article.
5. What is the most interesting thing you learned from this article?
6. List all of the words that you do not know.
This is due on Monday in class.
Tuesday, November 2, 2010
Layers of the Atmosphere
 Today we made a scale model of the layers of the atmosphere. For homework you need to finish your model and include three facts about each layer of the atmosphere on your model.
Today we made a scale model of the layers of the atmosphere. For homework you need to finish your model and include three facts about each layer of the atmosphere on your model.Homework part 2: Watch the video clip below about the atmosphere from National Geographic.
Monday, November 1, 2010
New Unit: The Air
These are all questions we'll answer over the next few weeks. Today we learned how elements and compounds relate to the air. For homework: Read and take notes on the layers of the atmosphere. Click the picture to link to your homework assignment. You will have a reading quiz on this information on Tuesday.

Please bring the lid of a shoe box to class tomorrow for a mini-project.
Monday, October 25, 2010
Test Today
Friday, October 22, 2010
Test Review
Make sure to review all your notes and handouts from class.
Wednesday, October 20, 2010
Candle Lab
 Homework: if you didn't finish the analysis questions in class, please finish these for homework. On Friday we will be wrapping up our water unit and reviewing for the test on Monday.
Homework: if you didn't finish the analysis questions in class, please finish these for homework. On Friday we will be wrapping up our water unit and reviewing for the test on Monday.
Monday, October 18, 2010
Boiling Point Web Lab

So, we have a change of plans.
Tomorrow we will do the candle lab and organize your notebook for your notebook check on Monday.
Homework: make sure you bring your notebook to class and if your notebook is a mess, dig through your room and find all of your science papers; BRING THESE TO CLASS!.
States of Matter & Phase Change

Homework: Read about the differences between chemical and physical changes here. Take notes for a reading quiz tomorrow.
If you have a computer or iPad, please bring it to class tomorrow.
Friday, October 15, 2010
Club Significance

Today we started to learn about significant figures and how to identify them in our calculations.
For homework you need to do problems 1-10 on the front of the worksheet. You do not need to do the problems on the back, we will do those in class on Monday.
Thursday, October 14, 2010
Density of Water
To look at density we attempted the soda can demo. What we should have seen was this:

Instead we "defied" the laws of density :) Maybe Swiss Coke is different from American Coke...
Tomorrow we will be going back over the data you have collected in the density experiments to determine the how to accurately present our data using significant figures.
Tuesday, October 12, 2010
Revisiting the Density Cubes
 Homework: None. However there was a bonus homework question proposed by Gianromano: "Why is the formula for density mass divided by volume and not volume divided by mass?" Find out the answer and get a bonus point on your homework.
Homework: None. However there was a bonus homework question proposed by Gianromano: "Why is the formula for density mass divided by volume and not volume divided by mass?" Find out the answer and get a bonus point on your homework.
Monday, October 11, 2010
Lab Check Up!
Over the weekend there was a mix up in the lab involving various materials. The cubes you are about to receive were all labeled neatly in containers. However, something happened and now we don’t know what the cubes are made out of anymore. The science department needs your help finding the density of these substances to determine what they are.
Homework: Click the picture below and it will take you to a website. Click on the following areas, read about them and take notes. You will use your notes for a mini open notes quiz tomorrow.
 You should read about AND take notes on: Chemical, Nutrients and Suspended Matter.
You should read about AND take notes on: Chemical, Nutrients and Suspended Matter.Friday, October 8, 2010
Finding Mass Using a Triple Beam Balance

Homework: First, use the handout below from the Day 1 of Volume by Displacement. Use the volume you found for four of your objects and use the mass you found from today's activity, to find the density of each object.

For Example: If you found the volume of Rock #22 to be 30mL and the mass of Rock #22 to be 10g, the density would be 0.33g/mL.
Wednesday, October 6, 2010
Volume by Water Displacement 2
Homework: You can either watch the video clip below AND take notes.... OR... go to this website and take notes. Your choice. Make sure you include the formula for finding density and the definition of density.
Tuesday, October 5, 2010
Volume by Water Displacement
Tomorrow we will evaluate our results and see if our data is accurate. There is no homework tonight.
Monday, October 4, 2010
Measuring Liquid Volume
Not everyone had the same number of drops fit on their coin. If all of the coins were about the same size, how is this possible? How can we make the measurements the same?
Today students learned about various tools that scientists use to take accurate measurements. Students did a worksheet to help them practice reading various volumes of liquid. Tomorrow we will be doing a wet lab using displacement to help us determine the volume of irregularly shaped objects.

Homework: Complete the worksheet for finding the volume of a regularly shaped solid. Note: you can absolutely use a calculator for this assignment.
Friday, October 1, 2010
Sinkin' Lincoln Lab
After the quiz we did the Sinkin' Lincoln Lab to examine surface tension. We also reviewed how to take an average and find the max./min. of a set of data.
 Homework: (due Monday): Finish the questions on the back of the lab sheet.
Homework: (due Monday): Finish the questions on the back of the lab sheet.
Wednesday, September 29, 2010
Water Jigsaw
Just to make sure that students were listening to their peers, we will have a 10 minute open notes quiz on Friday based on what we talked about in class on Wednesday. So...
Make sure you bring your notebook to class on Friday!
Tuesday, September 28, 2010
Properties of Water
When you take notes you can write in bullet points or complete sentences. If you type your notes, you MUST bring a paper copy to class. You can also use diagrams to help enhance your notes.
Some of these are note traditional readings, some of these websites are interactive or video clips.
Carlo
Malcolm
Richard
Francesca
Paul
Adam
Jose
Antonella
Gabriella
Gianromano
Isabella
Ivan
Jonatan
Tomi
Edo
Wednesday, September 22, 2010
Test Review
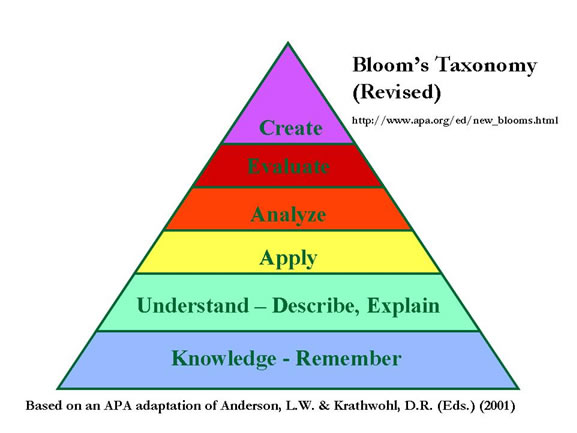
For your first test, 60-65% of the questions will be at the knowledge level. Our goal as the year progresses is to have less questions at the knowledge/remember level and more questions at the higher levels.
Tuesday, September 21, 2010
Eewww! That smells gross!

We also continued to practice how to identify controls and variables in an experiment. You have no written homework for tonight, other than to study for your test on Friday. Tomorrow we will be reviewing for your test.
Monday, September 20, 2010
Soil Bacteria and Controls and Variables
 After setting up our experiment, we used the Simpsons to practice identifying controls and variables in experiments.
After setting up our experiment, we used the Simpsons to practice identifying controls and variables in experiments.Homework: finish the back of the Simpsons controls and variables worksheet. Note: These two problems are harder than the three on the front of the worksheet. Just try your best. You can do it!
Here's a math hint for the Krusty the Clown problem:
50% (or half of 30) =15
So... if the new itching power is advertised to last for 50% longer that the original itching powder (which lasts 30 minutes), the new itching powder lasts for 45 minutes, then the advertisement is correct.
Sunday, September 19, 2010
Study Guides for Your Test on Friday
If your name is listed below you are staying in H period science. If your name is not listed below, you will have science with Mr. Ogilvie during B period.
Carlo
Malcolm
Richard
Francesca
PaulAdam
Jose
Antonella
Gabriella
Gianromano
Isabella
Ivan
Jonatan
TomiEdoardo
Below are the study guides for your test on Friday on the soil. Click the pictures to make them larger. The picture will open and be the size of a word document. There is a list of questions and a list of vocabulary.


Friday, September 17, 2010
Food Webs
 Homework: Read about Bacteria, Fungi and actinomycetes here. For each topic: Bacteria, Fungi and Actinomycetes write down three things you learned and two questions you have. This means you should have a total of 15 complete sentences. You must write in your own words. If you choose type this assignment, you must bring a printed copy to class.
Homework: Read about Bacteria, Fungi and actinomycetes here. For each topic: Bacteria, Fungi and Actinomycetes write down three things you learned and two questions you have. This means you should have a total of 15 complete sentences. You must write in your own words. If you choose type this assignment, you must bring a printed copy to class.
Tuesday, September 14, 2010
Berlese Funnels and Dichotomous Keys
We also learned about Dichotomous Keys and created our own for the class. For homework, due on Friday, you will need to make a dichotomous key for the organisms pictured below. There are lots of different ways to make a key, so it's OK if yours is different from someone else's key. Please work on your own to complete this assignment. You do not need to print out the pictures, just write your key in your notebook and bring it to class on Friday.

Monday, September 13, 2010
Element Sharing and Experimental Design
Tomorrow we will be making Berlese funnels and begin talking about how we will classify what we find.
 The illustration above shows how it works: a funnel (E) contains the soil (D), and a heat source (F) such as an electric lamp (G) heats the litter. Animals escaping from the dehydration of the soil descend through a filter into a preservative liquid (A) in a receptacle (B).
The illustration above shows how it works: a funnel (E) contains the soil (D), and a heat source (F) such as an electric lamp (G) heats the litter. Animals escaping from the dehydration of the soil descend through a filter into a preservative liquid (A) in a receptacle (B).Homework None:
Friday, September 10, 2010
Interpreting Nutrition Labels
Read the post below this one (or click here) for details about your Element Trading Card Assignment.
Today one of your peers asked, "But what do nutrition labels have to do with soil?! Why are we doing this?" You might have been thinking the same thing. We need elements, compounds, mixtures and molecules to function. Today we learned about some of these such as Vitamins A & C, Iron, Calcium, fat, sugar and protein. Next week we will learn about what elements, compounds, mixtures and molecules (ECMM) are in the soil. What ECMM do the organisms that live in the soil need? Are these the same ECMM that we need? Stay tuned!

We also looked at the molecular structure of fats, sugars and proteins, it turns out they a combination of H, C, O and sometimes N. What makes each molecule special? It's shape!
Thursday, September 9, 2010
The Periodic Table
Tomorrow in class you will each be given an element to make an 'element trading card.' These 'cards' will be due on Monday September 13th. The guidelines for the assignment are below. You can click on the picture to make it larger. You will also receive a copy of this paper tomorrow and your assigned element.
 Lastly, you must cite your sources. On the back of your trading card list all of the websites, books or other materials you used to gather your information. For example:
Lastly, you must cite your sources. On the back of your trading card list all of the websites, books or other materials you used to gather your information. For example:If your element were Zinc and you used this website, you would need to write the following information:
Name of the website, url, date you visited the website.
Chemical Elements.com - Zinc (Zn) http://www.chemicalelements.com/elements/zn.html, visited on (whatever day you visited the website).
Not sure where to find the name of a website or the URL? Click the picture below.

Tuesday, September 7, 2010
Elements, Compounds & Mixtures
 Homework:
Homework:1. Answer the question on the last page of your worksheets from today, "what elements do you think are found in the soil."
2. Using the periodic table in your student planner, identify the key elements 1-6 on the last page of the worksheet from today.
Soil Jigsaw

Homework: Complete the soil question sheet. No computers needed for this assignment.
Monday, September 6, 2010
What sound does a raisin make?

In our first unit, The Soil, we will be looking at something that many of us consider very ordinary.
Your first homework assignment is to find your name below and click on the link. On a sheet of paper
1. Write your name
2. The url for the website (a url is the address, for example: www.tasis.ch is a url)
3. Read the information on the webpage.
4. Write down three things you learned about soil. Be specific and please use complete sentences.
5. Two things you have questions about from the reading. These might be things you don't understand or things you want to learn more about.
This is assignment should take you no more than 20 minutes.
Carlo
Malcolm
Conner
Svetlana
Mauro
Paulina
Molly
Joo Hwan
Francesca
Paul-Wilhelm
Adam
Jose
Antonella
Gabriella
Gianromano
Isabella
Ivan
Oliver
Hayden
Jillian
Edoardo
It is VERY important that you do your homework, because we will be using this information in class tomorrow.
Sunday, September 5, 2010
Syllabus
 We will be starting our year learning about the soil. This is where you might be thinking: Huh? What? Ugh, really? Before you try and say that soil might be the most boring thing you’ve ever heard of, I ask you to think about this:
We will be starting our year learning about the soil. This is where you might be thinking: Huh? What? Ugh, really? Before you try and say that soil might be the most boring thing you’ve ever heard of, I ask you to think about this:Since [the beginning of] time humans and other animals have been dying of all manner of diseases and then buried in the soil, yet not a single major disease is transmitted by it." (Hillel 1991, p. 24).
So, what is it about the soil that makes it so unique? Both the air and the water can spread disease, why doesn't the soil spread disease?
Honesty: Being honest with yourself, is there something you don't understand? Ask. Being honest about your class and homework. Did you work on your own or did you copy from someone else?
Homework: Homework will be assigned almost every night and be incorporated into the following day’s class. A late assignment defeats the purpose of completing it. Therefore, late homework will not be accepted and will be graded as a zero. Assignments include, but are not limited to: worksheets; problem sets; readings; preparing for a discussion/presentation etc.
Make-up Work: You are responsible for obtaining missed assignments. If you know in advance you will be missing class for co-curriculars, an appointment etc. you should notify me in advance at least 2 days in advance.
Late Work Policy: As previously stated, late homework is not accepted. Late projects will be accepted up to three calendar days after the due date. However, each day that the project is late, 10% of the grade will be lost. After the third day the project will no longer be accepted.
Extensions: Extensions will not be given except in the event of extenuating circumstances, so plan your time wisely.
Attendance: Attendance is mandatory at all class meetings. You are expected to arrive on time in be in your seat with your homework and your notebook on your desk. A pattern of tardiness will be regarded as not meeting the expectations of the class and will affect your effort grade.
Class participation: In order to make the most progress in this class you will need to participate actively. Participating in class not only means sharing your ideas but also coming prepared and on time.
Notebook: Must be divided into two sections and have a table of contents:
You must keep a detailed table of contents for your notebook.
Class Rules:
· BE ON TIME!
· BE PREPARED! Bring the textbook, your notebook, highlighters and pens/pencils each day.
· BE PRESENT! Both in mind and body. Showing up for class doesn’t mean much if you are daydreaming.
· If you have an unexcused absence from class on the day of a test or quiz you will receive a zero.
· As soon as you need extra help, come find me. Don’t wait!
· NO eating or chewing gum in class. You may bring water.